Canada Finalizes Changes to Nutrition Labelling Requirements for Packaged Foods
Canada Finalizes Changes to Nutrition Labelling Requirements for Packaged Foods
In late October 2016, the federal Minister of Health launched the Healthy Eating Strategy intended, in part, to make healthier food choices easier for Canadians. In support of the Strategy, final amendments to the Food and Drug Regulations – Nutrition Labelling, Other Labelling Provisions and Food Colours were published in the Canada Gazette, Part II on December 14, 2016. These amendments focus primarily on changes to the Nutrition Facts table (NFt) and list of ingredients on packaged foods.
Nutrition facts table changes
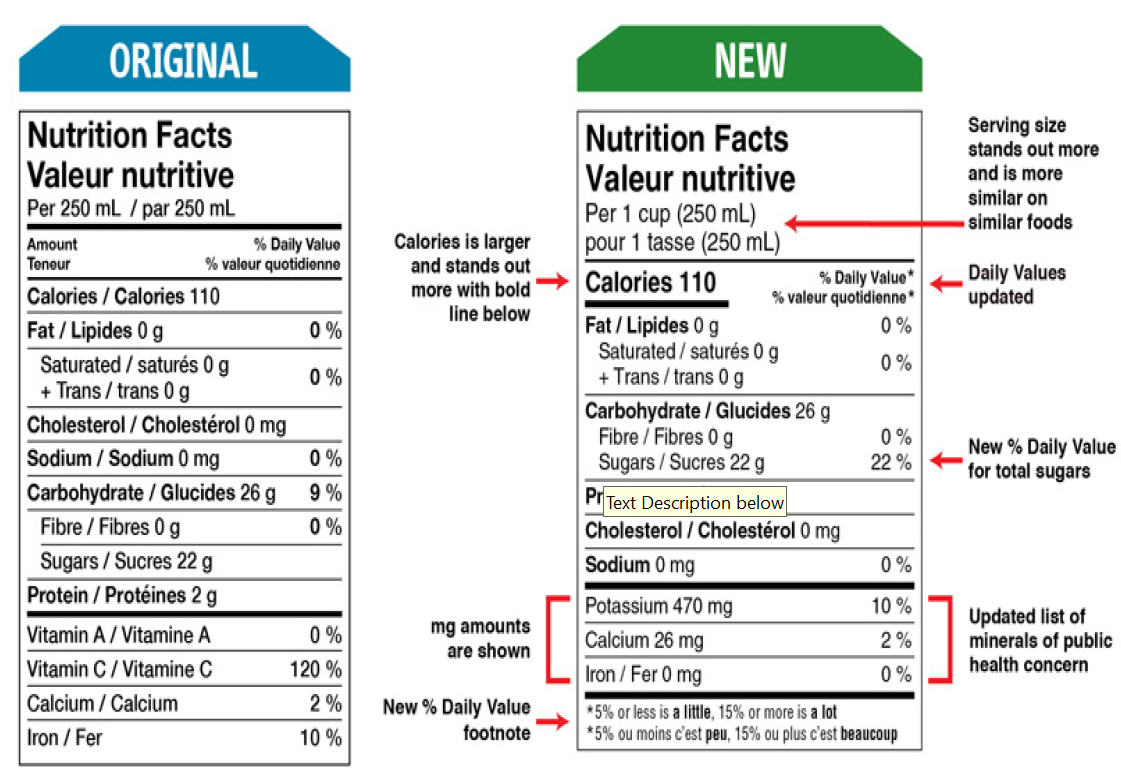
The NFt will now make serving sizes more consistent to allow similar foods to be compared more easily and help consumers better understand the amount of a food typically consumed in one sitting. Formatting changes[1] to the NFt are intended to make information on serving size and calories easier to find and read (the font size for serving size and calories will be increased, and a bold line added under the calorie information). A new percentage daily value for total sugars will also be added to the NFt, and the list of nutrients updated to add potassium (and require that the amounts of potassium, calcium and iron contained in the food be disclosed in milligrams) and remove vitamins A and C from the NFt. In addition, a footnote at the bottom of the NFt will provide context to daily value information by helping consumers understand how much sugar and other nutrients (such as sodium) are in the food, explaining that “5% or less is a little” and “15% or more is a lot”.
List of ingredients changes
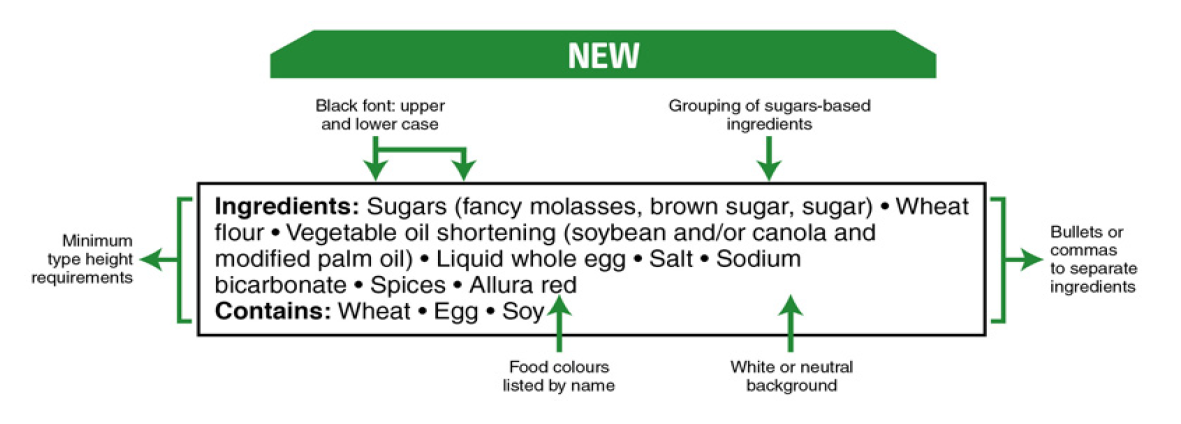
Changes to the list of ingredients include grouping sugars-based ingredients in brackets after the name ‘sugars’ to identify all of the sources of sugars added to a food and listing food colours by their individual common names. Formatting changes[2], including presenting the text of the list of ingredients in black font on a white or neutral background, providing minimum type height requirements for ingredients, using bullets or commas to separate ingredients, and using both upper and lower case letters for ingredients in the list are all aimed at making the list of ingredients easier to find, read and understand. The same formatting changes will also apply to any ‘contains’ statement within an ingredient list indicating the presence or potential presence of priority food allergens, gluten sources and added sulphites.
Serving size changes
As noted above, changes to serving size information within the NFt will be based on regulated reference amounts to more accurately reflect the amount of a food eaten in one sitting. Serving sizes will also be more consistent, making it easier to compare similar foods and know how many calories and nutrients are being consumed. These changes will affect both single-serve and multi-serving packages.
On single-serving packages containing up to 200 percent of the reference amount for that food, the serving size will be the amount in the whole container. On multi-serve packages, serving sizes will be in an amount as close as possible to the food’s reference amount and will be based on the type of food, such as foods that can be measured, foods that come in pieces or are divided, and amounts of foods that are typically eaten.
For foods that can be measured, the serving size will be shown as a common household measurement, such as a cup, teaspoon or tablespoon. This will be paired with its metric equivalent in millilitres or grams. Similar products will have the same millilitre or gram amount, making them easier to compare. For foods that come in pieces, like crackers, or are divided into pieces before eating, like pizza, the serving size will be shown as either the number of pieces or as a fraction of the food. This will be paired with its weight in grams. Similar products will have the same or very similar gram amounts. For certain foods, like sliced bread, the serving size will reflect the way they are typically eaten (2 slices of bread), followed by the weight of the food in grams.
Sugars information
Changes to sugars include a percentage daily value for total sugars included in the NFt to help allow for comparison of the sugars content of different foods and identification of foods high in sugar, such as those with a sugars daily value of 15 percent or more. In the list of ingredients[3], sugars-based ingredients will now be grouped in brackets in descending order by weight after the name ‘sugars’ so that information with respect to the sugars that have been added to the food, the sources of sugars added to food, and how much sugars are added to the food in contrast to other ingredients will be more apparent.
New health claim permitted
The amendments also introduce a new health claim – ‘A healthy diet rich in a variety of vegetables and fruit may help reduce the risk of heart disease’ – that will now be permitted on vegetables and fruit as specified within the regulations.
Five-year transition
A five-year transition period for industry to come into compliance with the new requirements is provided within the amendments. During this transition period, those affected are permitted to follow either the former or the new labelling requirements.
by Janine MacNeil
[1] Government of Canada, Food Labelling Changes (Ottawa: Government of Canada, 2016), online: http://www.healthycanadians.gc.ca/eating-nutrition/label-etiquetage/changes-modifications-eng.php.
[2] Supra note 1.
[3] Supra note 1.
A Cautionary Note
The foregoing provides only an overview and does not constitute legal advice. Readers are cautioned against making any decisions based on this material alone. Rather, specific legal advice should be obtained.
© McMillan LLP 2016
Insights (5 Posts)View More
Ontario (Might get the) Right to Repair – An overview of Bill 187 the Right to Repair Consumer Electronic Products, Household Appliances, Wheelchairs, Motor Vehicles and Farming Heavy Equipment Act, 2024
Ontario considers new right to repair legislation for consumer products and motor vehicles.
More Than Meets the Eye: The Legal Implications of British Columbia’s Agreement to Recognize Aboriginal Title Over Haida Gwaii
An analysis of legal implications related to the BC Government's agreement with the Haida Nation to recognize Aboriginal title over Haida Gwaii.
Lessons Learned from the TTC’s Ransomware Attack
Lessons learned from the recent investigation by the Ontario IPC into the effectiveness of the TTC's cybersecurity measures and ransomware attack response
Don’t Get Caught by Canada’s Patent Novelty Grace-Period
The key difference between Canada and other jurisdictions like the United States when relying on the grace-period for inventor disclosures.
Shifting Gears – Canada to Consider New Motor Vehicle Equipment Regulations to Help Prevent Auto Theft
Transport Canada announces plan to update safety standards to combat auto theft.
Get updates delivered right to your inbox. You can unsubscribe at any time.